
FAQ
About the verification system and its operation
What is the medicines verification system?
The European Medicines Verification System (EMVS) is a unified pan-European system that prevents falsified medicines from reaching patients through legal medicine supply chain, e.g., via pharmacies or healthcare institutions. The Latvian Medicines Verification System (LZVS) has been established and is maintained by the Latvian Medicines Verification Organisation (LZVO).
What defines the implementation of the system?
The basis for the establishment of the medicines verification system is the Falsified Medicines Directive (2011/62/EU) and the Delegated Regulation which defines detailed rules for the safety features appearing on the packaging of medicinal products for human use (EU 2016/161).
How does the system impact consumers?
Consumers can feel safe buying medicines at a pharmacy or receiving medicines in healthcare institutions.
Who supervises and controls the system?
There are three competent institutions in Latvia:
- the Ministry of Health develops health policy and legislation, including the base of regulatory acts for the circulation of medicines and the operation of the medicine verification system in Latvia.
- the State Agency of Medicines registers medicines, coordinates the placement of safety features on the packaging of medicines registered in Latvia and imported in parallel.
- the Health Inspectorate monitors the circulation of medicines, investigates suspected falsification, and controls compliance with the order of distribution of medicines, as well as generally supervises the verification process of medicines.
How does the medicines verification system work?
The European Medicines Verification System (EMVS) consists of the European central repository (EU Hub) and National Medicines Verification Systems (NMVS). Manufacturers and parallel importers upload their unique identifiers via the European Hub and verification of medicines take place in NMVS, where the uploaded data for relevant markets are stored.
All EU countries (including EEA countries) have their own NMVS (except for Belgium and Luxembourg, where there is a supranational medicines verification system). NMVS are used by local members of the medicines supply chain, i.e., wholesalers, pharmacies, hospital pharmacies and other healthcare institutions, who verify authenticity of each pack and decommission the unique identifier of the medicinal product.
What is a unique identifier?
The unique identifier is the safety feature enabling the verification of the authenticity and the identification of an individual pack of a medicinal product. Manufacturers encode the unique identifier in a two-dimensional barcode of each pack during the production process. The unique identifier is decommissioned when the pack is supplied to the public.
The unique identifier is a sequence of numeric or alphanumeric characters that is unique to a given pack of a medicinal product. The unique identifier consists of the following data elements:
- the product code (GTIN/NTIN),
- the batch number,
- the expiry date,
- the serial number.
The product code consists of the data elements allowing the identification of the name, the common name, the pharmaceutical form, the strength, the pack size, and the pack type of the medicinal product.
Manufacturers encode the unique identifier into a two-dimensional barcode (2D code), which is a machine-readable data matrix. The code is scanned and compared to the unique identifiers stored in the medicines verification system to verify the authenticity of the medicine.
What is a serial number?
The serial number is part of the unique identifier and is unique to each pack of medicine.
It consists of a numeric or alphanumeric sequence of maximum 20 characters, generated by a randomization algorithm. The probability that the serial number can be guessed shall be negligible and lower than 1 in 10,000. The character sequence resulting from the combination of the product code and the serial number is unique to a given pack of a medicinal product until at least one year after the expiry date of the pack or five years after the pack has been released for sale or distribution, whichever is the longer period.
About the medicines verification process
Do all medicines need to be verified?
All prescription medicines and the over-the-counter medicine omeprazole must be verified. The whitelist and blacklist are available in Annexes I and II of the Delegated Regulation (EU) 2016/161 made by the European Commission on October 2, 2015.
Which medicines do not need to be verified?
Over-the-counter medicines, except for omeprazole, as well as all food supplements (such as vitamins, etc.) and medical supplies, do not need to be checked within the verification system. There are also certain exceptions for prescription medicines, such as various radionuclides, medicinal gases, certain intravenous solutions, contrast media, and homeopathic medicinal products. Please check the full list of exceptions in Annex I of the Delegated Regulation.
What is the difference between verification and decommissioning?
Two basic transactions are possible in the verification system – verification and decommissioning.
During the verification process the unique identifier of the product is checked against data held in the national verification system to verify that the product is authentic and is available for supply to the patient. Verification is a process that can be done at any time during distribution process of the medicine through the supply chain.
Decommissioning consists of two processes in one. First a pack is verified and, if successful, then a pack is decommissioned. Usually by decommissioning transaction the status of the pack changes from ‘active’ to ‘supplied’ that means the pack has been dispensed. There are also other decommissioning states available, e.g., ‘exported’, ‘sample’, ‘free sample’, ‘locked’, ‘destroyed’ and ‘stolen’. Transactions ‘destroyed’ and ‘stolen’ are terminal states, meaning that they cannot be reversed.
Who verifies and who decommissions the medicines?
All participants of the supply chain, except end users, only verify the packs against the database. The pack is checked and simultaneously decommissioned from the system only by the end user when the medicinal product is supplied to the public – sold (at a pharmacy) or used (by the healthcare professionals) for a patient.
In accordance with the Regulations No. 416 of the Cabinet of Ministers of the Republic of Latvia, wholesalers verify the safety features and decommission the unique identifier of a medicinal product in the Latvian Medicines Verification System before they supply vaccines to family doctor's practices and paramedics; before they supply medicine to social healthcare institutions; veterinarians, prisons, ambulance, persons who have a permit for the purchase of medicines issued by the State Agency of Medicines in accordance with the first part of Article 48 of the Pharmaceutical Law; healthcare institutions of the Ministry of Defence (National Armed Forces), with the purposes of civil protection and disaster control.
When does the system generate an alert?
When a pack is scanned in pharmacy or healthcare institution, it could generate an alert if the data submitted is not correct and does not match the data stored in the NMVS. When a pack is in a decommissioned state, it cannot be further decommissioned. It will generate an alert if a repeated decommission transaction is attempted.
Most alerts are technical in nature, arising either on the side of manufacturers (incorrectly entering data about the product, pack, lot, expiration date or market) or on the side of end user, using misconfigured scanners or systems or not following verification rules, for example by repeatedly scanning a single pack. This is a common mistake if pharmacy supply to consumer only a part of the pack of a medicinal product.
About the maintenance and data security
Who funds the medicines verification system?
The manufacturers finance the implementation and maintenance of the European and National Medicines Verification Systems. The regulation stipulates that the pharmaceutical manufacturers cover most of the expenses. They invest in the improvement of the manufacturing lines, enabling the labelling of each packaging of medicinal products.
How is data security ensured?
Legislation strictly states that the data in the system belongs only to the entity that generates it. Supply chain members do not have access to data created by other members. The system does not store personal data.
Who can connect to the system?
The Latvian Medicines Verification System (LZVS) may be accessed and used only by end users whose identity, role, and legitimacy have been verified and accepted. End users may access and use the LZVS only to verify and/or decommission the unique identifier of the medicinal product that is under their physical possession.
Can the system be used for other purposes?
No, currently the medicines verification system is not intended for other purposes than those stipulated in Falsified Medicines Directive (2011/62/EU) and the Delegated Regulation (EU 2016/161). The use of data collected in the verification system currently is not allowed for e.g., tracking medicines packs or preventing medicines shortages.
About end users of the verification system
Who needs to connect to the system and verify the medicine?
The obligation to verify medicines in Latvia is set for all pharmacies (hospital and public), wholesalers, hospitals, healthcare institutions that have permission to purchase medicines, e.g., polyclinics, health centres, doctors' practices, as well as dental practices and clinics.
When should the pack be decommissioned from the system?
Persons authorised or entitled to supply medicinal products to the public should verify the authenticity and decommission a unique identifier at the time the medicinal product is supplied to the public.
Persons authorised or entitled to supply medicinal products to the public operating within a healthcare institution may carry out that verification and decommissioning at any time the medicinal product is in the physical possession of the healthcare institution, provided that no sale of the medicinal product takes place between the delivery of the product to the healthcare institution and the supplying of it to the public.
How many end users are in Latvia?
The number of end users is constantly changing, as the Latvian Medicines Verification Organisation (LZVO) ensures that only legitimate users are granted access to the system. Currently, approximately 1,210 end users have connected to the Latvian Medicines Verification System.
About end users’ obligations
What are responsibilities of the end users?
End users, subject to the verification obligation, must sign an agreement with the Latvian Medicines Verification Organisation (LZVO) and connect to the system. End users must choose their IT service provider, which has developed a solution to connect to the Latvian Medicines Verification System (LZVS).
End users must provide themselves with all the necessary equipment, services, facilities, and software to use the LZVS, including the relevant computer equipment and an Internet connection. End users may access and use the LZVS only to verify and/or decommission the unique identifier of the medicinal product that is under their physical possession.
What to do if the system does not work?
Where technical problems prevent end users from verifying the authenticity of and decommissioning a unique identifier at the time the medicinal product bearing that unique identifier is supplied to the public, those persons shall record the unique identifier and, as soon as the technical problems are solved, verify the authenticity of and decommission the unique identifier.
Technical problems could arise from an internet interruption, the unavailability of an IT system, an electricity supply interruption, or other technical issues on the side of the end user or on the side of the Latvian Medicines Verification System.
When to decommission only part of a pack?
The medicines verification system does not change anything in the Latvian regulation regarding supply of only part of the pack of a medicinal product. Where end users supply only part of a pack of a medicinal product the unique identifier of which is not decommissioned, they shall verify the safety features and decommission that unique identifier when the pack is opened for the first time.
Each end user must follow their own internal guidelines and procedures to remove only part of the pack of a medicinal product from their inventory accounting/record keeping system.
What to do if a pack has been decommissioned by mistake?
If the end user decommissioned the pack from the system by mistake, he/she must revert the status of a decommissioned unique identifier to an active status within 10 days.
Medicinal products bearing a unique identifier which cannot be reverted to an active status because the conditions set out in the legislation are not fulfilled (e.g., more than 10 days; other location; product expired, recalled, withdrawn, intended for destruction, or stolen) shall not be returned to saleable stock. ‘Undo’ transaction will fail with the system indicating that an L1-4 error has occurred.
About the alert investigation
What to do if an alert is raised during decommissioning?
The medicine that triggered the alert must not be sold to or used by patients. This medicine should be quarantined until the cause of the alert is resolved. When the alert is generated in the system, it should be investigated by the end user who generated the alert and the manufacturer/Marketing Authorization Holder of the specific medicine’s pack. The purpose of the investigation is to rule out that the alert triggered in the system has been caused for technical reasons, such as issues with the repository system, data upload, data quality, incorrect end-user scanning or other similar technical issues.
Authorities should be informed as soon as the alert triggered in the system cannot be explained by technical issues with the verification system, the data upload, the person performing the verification or similar technical issues. The Health Inspectorate is the responsible state institution in Latvia who should be informed in case of potential falsification.
Where to find information about alert investigation?
The European Medicines Verification Organisation (EMVO) has approved and published the "Best Practice on Alert Handling Guideline", which describe the general principles for the investigation of alerts and the flow of information, as well as define all parties involved in the process – EMVO, National Medicines Verification Organisation (NMVO), the manufacturer and the obligations and rights of system end users in the process of processing alerts. The guidelines available here.
Alert return codes’ description
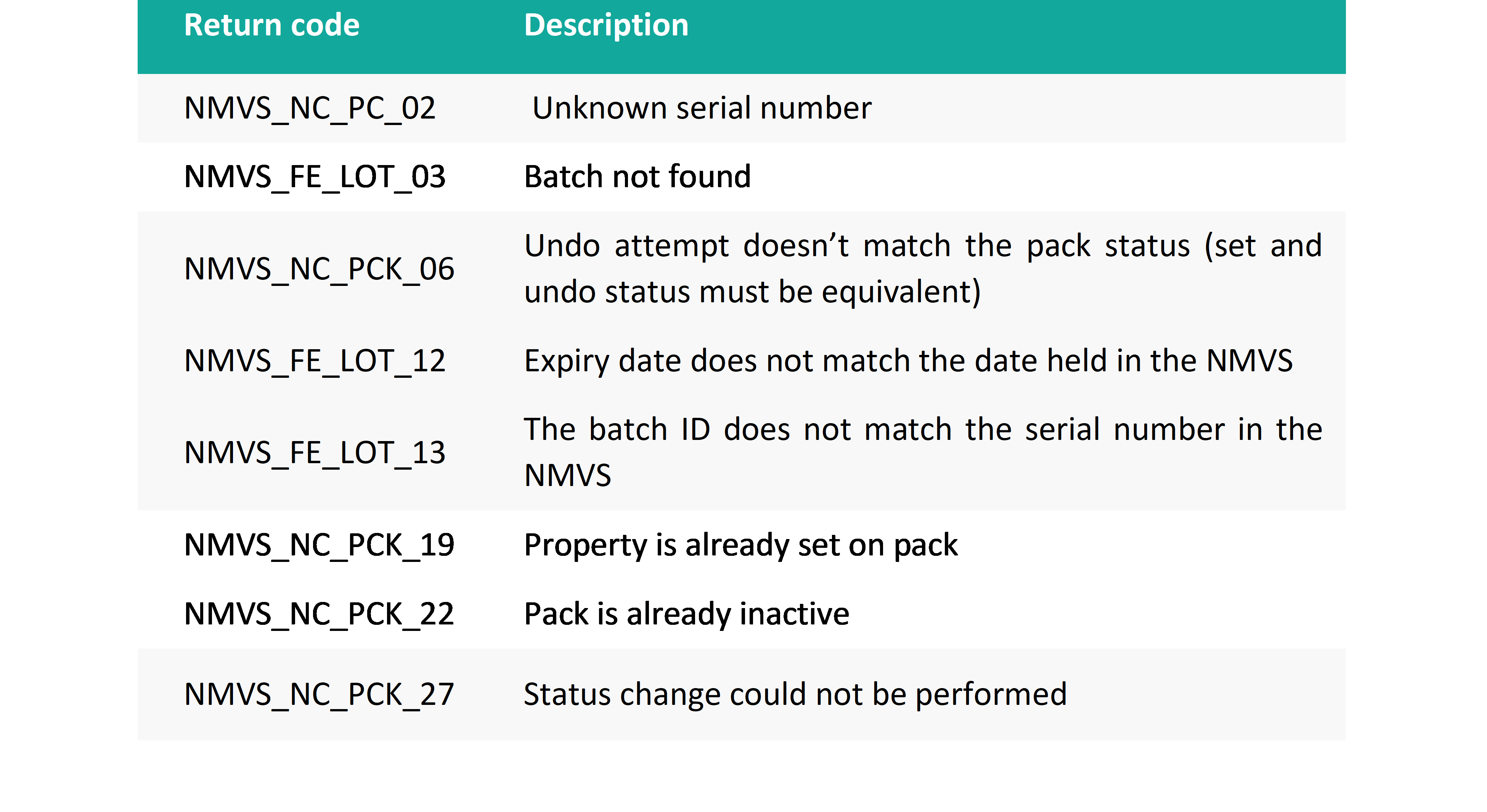
What should end user do in case of alert?
A medicine’s pack that has generated an alert should not be sold or used by a patient. When the verification system generates an alert, both the end user and the manufacturer should initiate a simultaneous investigation. The end user performs the following actions:
- the medicine’s pack must be quarantined/ withhold pack from saleable stock until the cause of the alert is resolved,
- make sure that the cause of the alert is not a technical, procedural, or manual error,
- if the alert is not identified on the end user's side, then the investigation of the alert should be started by contacting the marketing authorisation holder (MAH),
- the marketing authorisation holder, the Latvian Medicines Verification Organisation or the Health Inspectorate may request a photo of the medicine pack that caused the alert for further investigation,
- the alert and its investigation must be documented,
- if necessary, the end user can ask for the support of the Latvian Medicines Verification Organisation and/or the Health Inspectorate.
What should MAH do in case of alert?
When the verification system generates an alert, both the end user and the manufacturer should initiate a simultaneous investigation. The Marketing Authorisation Holder (MAH) investigates the alert by performing the following steps and checking:
- are there any data upload errors?
- is there an error in the EU Hub?
- communicates with the Latvian Medicines Verification Organisation about possible causes,
- requests a photo of the pack from the end user,
- in special cases, the pack can be requested from the end user.
If the cause of the alert is found, appropriate corrective measures must be taken, for example, the data upload error must be corrected, and the end user and the Latvian Medicines Verification Organisation must be informed.
Which alerts MAH may not investigate?
According to the European Medicines Verification Organisation (EMVO) guidelines "Best Practice on Alert Handling Guideline", Marketing Authorization Holders (MAH) are not required to investigate A7, A24 and A68 alerts, except in the following circumstances:
- MAH is aware they have caused the alert(s) due to repeating decommissioning transactions when packs are under their control,
- an end user contacts MAH about specific alert(s),
- the NMVO contacts MAH about specific alert(s), e.g., in the case of an A7, A24 or A68 alert generated by an end user where no end user root cause can be identified,
- the national competent authorities (NCA) request MAH to investigate specific alert(s).
The reason for this approach is that A7 and A24 alerts generated by end users will rarely be due to errors on the part of the MAH. Similarly, the vast majority of A68 alerts generated by end users are due to end user software or scanner issues.
How long does it take for the MAH to respond?
MAH is required to provide feedback on their investigation to NMVO within 2 working days.
About the obligations of MAH
What are the main responsibilities?
Marketing Authorisation Holders must:
- ensure that all packs put on the market in Europe, including Latvia, after February 9, 2019, contain safety features consisting of a unique identifier and an anti-tampering device on the packaging of certain medicinal products for human use,
- connect to the EU central repository (EU Hub), which is managed by the European Medicines Verification Organisation,
- upload data about medicine packs to the EU Hub,
- conclude cooperation agreement with the Latvian Medicines Verification Organisation,
- make regular payments for the maintenance of the Medicines Verification System,
- investigate the alerts,
- inform the Health Inspectorate if falsified medicines are suspected.
When should a cooperation agreement need to be concluded?
The Marketing Authorisation Holder must conclude a cooperation agreement with the Latvian Medicines Verification Organisation as soon as it is planned to distribute the medicines on the Latvian market and/or data on product batches and packs are uploaded to the Latvian Medicines Verification System.
What needs to be done to conclude a cooperation agreement?
The Marketing Authorisation Holder (MAH) must contact the Latvian Medicines Verification Organisation and submit basic information for concluding the contract by sending it to the e-mail info@lzvo.lv:
- name of the contracting party;
- registration number of the contracting party;
- name, surname, e-mail, current position and phone number of one contact person;
- full name of the MAH;
- e-mail and telephone number of one contact person for investigation of alerts for each MAH.
How is the cooperation agreement concluded?
The cooperation agreement between the Latvian Medicines Verification Organisation (LZVO) and the Marketing Authorisation Holders has been created considering the requirements of the Falsified Medicines Directive and the Delegated Regulation regarding the co-financing of the cost of the Latvian Medicines Verification System (LZVS) and all other expenses related to the maintenance and operation of the LZVS. The standard contract has been approved at the General Assembly of LZVO. It is not negotiable. After harmonizing the details of the contract, the signatories of both parties sign it physically or with a secure electronic signature.
What is the fee?
The Latvian Medicines Verification Organisation (LZVO) is responsible for the development, implementation, operation, and maintenance of the Latvian Medicines Verification System. The Marketing Authorisation Holders (MAHs) undertake to finance costs related to the LZVO in accordance with the provisions of the agreement.
The contract provides for two types of fees: a one-time registration fee and an annual fixed flat fee. The amount of the fixed annual flat fee is variable from year to year. The size of annual flat fee is approved by the General Assembly of the LZVO, and the LZVO informs all MAHs about the amount of the fixed annual flat fee for the following year no later than November 30 of the previous year.
What to do if suspected falsification?
What is a falsified medicine?
Falsified medicine – any medicine with falsified:
- identity (packaging, labelling, name, composition, strength),
- source (manufacturer, country of manufacture, country of origin, owner of registration),
- history (manufacturing documents and documents related to the distribution networks used).
When should competent authorities be notified?
Immediately, on the day of discovery of the fact, wholesaler, pharmacy manager or Marketing Authorisation Holder must notify the Health Inspectorate if there is a suspected falsification.
What must be included in the notification?
According to the Cabinet of Ministers Regulation No. 416 "Procedures for distribution and quality control of medicines" Article 116, the notification to the Health Inspectorate must include:
- medicines name, strength or concentration and dosage form,
- information about the manufacturer and Marketing Authorisation Holder (MAH) (if any) indicated in the medicine’s label and instructions for use,
- serial number,
- the person (company) from whom the medicine was purchased,
- other information that helps to investigate a possible falsification (for example, an alert ID).
To whom must report suspected falsified medicines?
If there are suspicions of possibly falsified medicines and possibly low-quality medicines, Cabinet of Ministers Regulation No. 416 "Procedures for distribution and quality control of medicines" the persons referred to in Article 11 and 13, a merchant or an economic operator who has been issued a special permit (license) for the opening (operation) of a pharmacy, healthcare institutions, social care institutions, practicing veterinarians, veterinary healthcare institutions or other persons shall immediately, on the day of discovery of the fact, notify the Marketing Authorisation Holder (MAH) and the Health Inspectorate.
If the Health Inspectorate, upon receiving a notification, suspects the falsification of medicines or raw materials, the Health Inspectorate informs the law enforcement authorities.
How to store potentially falsified medicines?
Falsified medicines detected in the distribution chain are stored separately from other medicines to prevent any possibility of mix-up. They must be clearly marked with a special indication – “not intended for sale".
About the client portal
What is a client portal?
The Latvian Medicines Verification Organisation (LZVO) has created a client portal, which has two modules and is intended only for registered users – Marketing Authorisation Holders (MAHs) and end users of the system. The client portal has an individual profile for each LZVO cooperation partner or company, as well as direct access to professional information and convenient communication with the LZVO.
What information is available on the client portal?
The end users’ section of the client portal is online connected to the data from Enterprise Register, the State Agency of Medicines, and the Health Inspectorate. Therefore, the most up-to-date information related to the end users is available, that is very important to ensure access to the Latvian Medicines Verification System only for legitimate end users. It is the responsibility of end users to maintain the rest of the data in the client portal up to date, e.g., contacts persons for alert investigation and communication, their phone number and e-mail.
Marketing Authorisation Holders (MAHs) have access to the most up-to-date information in the client portal about flat fees, invoices, the single point of contact (SPOC) and the alert investigation person of the MAH, as well as about other cooperation issues.